Fordi det kunne betale sig for hospitalerne at bruge det. Og dermed drukne patienter 🙁
Det er en ret rystende video:

Her er linket til artiklen, der advarer mod remdesivir
Og samtidig gjorde myndighederne ALT for at hindre midler, som VIRKER 🙁

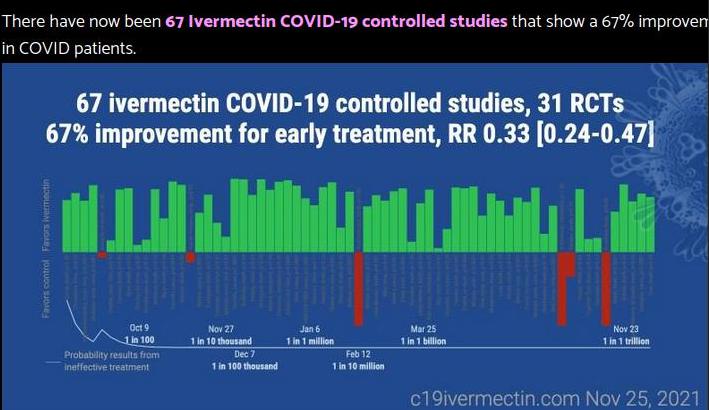
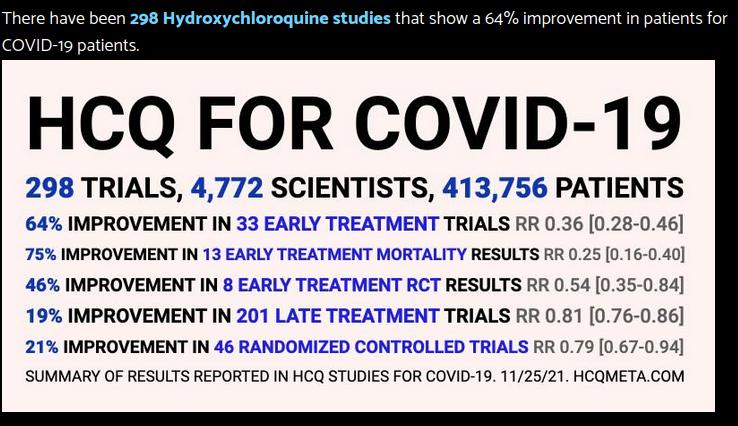
taget herfra og fra Vlad
Mig bekendt har også danske hospitaler brugt remdesivir, men jeg mener de er holdt op med det ?
https://rumble.com/vsa4ch-icu-doctor-every-covid-death-should-be-considered-a-murder.html
Se hos Aamunds blog og Snaphanen hvordan politikere er ved at udskifte civilisation og vestand med barbari og fattigdom
Man har en billig, afprøvet, ugiftig og lynhurtigt helbredende medicin, Ivermectin, der har haft kæmpesuccés i flere lande, Indien, Japan og Taiwan mfl., men det vil man ikke bruge fordi der er gået politik i sagen. Er politikerne i Vesten næsten alle røde landsbytosser?
https://gellerreport.com/2022/01/internal-hospital-data-confirm-huge-increase-in-patients-with-vaccine-side-effects-in-2021.html/
Se også kommentarerne.
Søg på El Salvador og Ivermectin
Landet sender nu medicinposer ud til borgerne – poser med bl.a. Ivermectin, vitamin C og D og andre piller
Meget mærkeligt. Den korte avis reklamerer i dag ivrigt for mere vaccinering. Det er gået helt hen over hovedet på den at der er store livsfarlige problemer med “vaccinerne”. Den korte avis er jublende begejstret for booster-stik osv. Ret uforståeligt.
Remdesivir er ikke på listen på ProMedicin:
https://pro.medicin.dk/Search/Search/SearchAlpha/r
Hej Hodja
hvad er konsekvensen af at Remdesivir ikke er på Promedicin ?
Betyder det at det ikke må bruges i Danmark ?
mvh TRumfEs
Jeg tror du skal ansøge om at bruge det til klinisk undersøgelse.
På danske hospitaler anvendes flere medikameter som kan give eller forværre nyresvigt. Det beror på en vurdering af gavnligheden i forhold til omkostningerne (for patienten) om man bruger det.
Nyresvigt er i almindelighed ikke dødeligt. Nyrerne kan erstattes med dialyse. Hos mange patienter med akut nyresvigt vender nyrefunktionen tilbage efter noget tid.
Monitoring and Adverse Effects
Remdesivir can cause gastrointestinal symptoms (e.g., nausea), elevated transaminase levels, an increase in prothrombin time without a change in the international normalized ratio, and hypersensitivity reactions.
Liver function tests and prothrombin time tests should be performed for all patients before they receive remdesivir, and these tests should be repeated during treatment as clinically indicated. Remdesivir may need to be discontinued if a patient’s alanine transaminase (ALT) level increases to >10 times the upper limit of normal, and it should be discontinued if an increase in ALT level and signs or symptoms of liver inflammation are observed.8
Considerations in Patients With Renal Insufficiency
Each 100 mg vial of remdesivir lyophilized powder contains 3 g of sulfobutylether beta-cyclodextrin sodium (SBECD), and each 100 mg/20 mL vial of remdesivir solution contains 6 g of SBECD.8 SBECD is a vehicle that is primarily eliminated through the kidneys. A patient with COVID-19 who receives a loading dose of remdesivir 200 mg would receive 6 g to 12 g of SBECD, depending on the formulation. This amount of SBECD is within the safety threshold for patients with normal renal function.9 Accumulation of SBECD in patients with renal impairment may result in liver and renal toxicities. Clinicians may consider preferentially using the lyophilized powder formulation (which contains less SBECD) in patients with renal impairment.
Because both remdesivir formulations contain SBECD, patients with an estimated glomerular filtration rate (eGFR) of <50 mL/min were excluded from some clinical trials of remdesivir; other trials had an eGFR cutoff of <30 mL/min. The FDA product label does not recommend using remdesivir in patients with an eGFR of <30 mL/min due to a lack of data.10 Renal function should be monitored before and during remdesivir treatment as clinically indicated.8
In 2 observational studies that evaluated the use of the solution formulation of remdesivir (not the reconstituted lyophilized powder formulation) in hospitalized patients with COVID-19, no significant differences were reported in the incidences of adverse effects or acute kidney injury between patients with an estimated creatinine clearance (CrCl) of <30 mL/min and those with an estimated CrCl of ≥30 mL/min.11,12 In 1 study, 20 patients had an estimated CrCl of <30 mL/min and 115 had an estimated CrCl of ≥30 mL/min;11 the other study included 40 patients who had an estimated CrCl of <30 mL/min and 307 who had an estimated CrCl of ≥30 mL/min.12 These observational data suggest that remdesivir can be used in patients with an eGFR of <30 mL/min if the potential benefits outweigh the risks.
https://www.covid19treatmentguidelines.nih.gov/therapies/antiviral-therapy/remdesivir/
https://www.ncbi.nlm.nih.gov/books/NBK553144/figure/article-25714.image.f1/?report=objectonly
https://www.ncbi.nlm.nih.gov/books/NBK553144/figure/article-25714.image.f2/?report=objectonly
https://www.ncbi.nlm.nih.gov/books/NBK553144/figure/article-25714.image.f3/?report=objectonly
Fra september 2021: https://www.ncbi.nlm.nih.gov/books/NBK553144/